
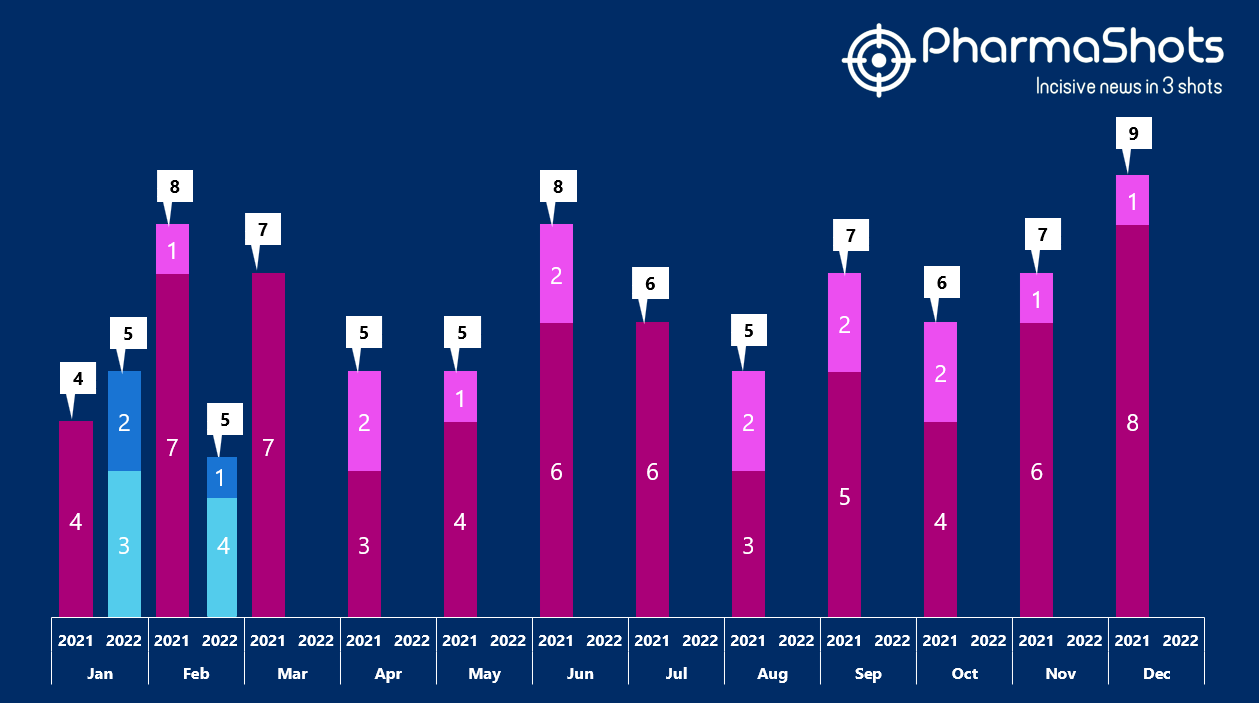
Insights+: The US FDA New Drug Approvals in February 2022
- The US FDA has approved 4 NDAs and 1 BLAs in Jan 2022, leading to treatments for patients and advances in the health care industry. The CDER and CBER approved 10 novel products in 2022
- In Feb 2022, the major highlights drugs were Enjaymo for cold agglutinin disease, Fleqsuvy for spasticity associated with multiple sclerosis, Pyrukynd for hemolytic anemia, NephroScan for kidney disease, Vonjo for myelofibrosis and thrombocytopenia
- PharmaShots has compiled a list of a total of 5 new drugs approved by the US FDA in february 2022
Enjaymo
Active ingredient: sutimlimab-jome Approved: February 04, 2022
Company: Sanofi Disease: Cold Agglutinin Disease
- The approval was based on the P-III (CARDINAL) study to evaluate Enjaymo in 24 patients with CAD. Patients continued on Enjaymo in an extension study upon the completion of 26wk. treatment period
- The study met its 1EPs & 2EP i.e., patients met the composite 1EPs criteria (54%), 63% achieved a Hgb ≥ 12 g/dL or an increase of 2g/dL; 71% remained transfusion-free after 5wk., 92% did not use other treatments & a mean increase in Hgb level @ 26wk., reduction in bilirubin levels
- The product will be available in the US in the coming wks. Enjaymo Patient Solutions provides access to eligible patients & enables disease education, financial, co-pay assistance programs & other support services
Fleqsuvy
Active ingredient: baclofen oral suspension Approved: February 07, 2022
Company: Azurity Disease: Multiple Sclerosis
- The US FDA has approved Azurity’s Fleqsuvy (baclofen oral suspension) as a concentrated formulation for the treatment of spasticity related to MS or patients with spinal cord injuries and other spinal cord diseases
- The approval was based on a bioavailability study to evaluate baclofen (oral tablet) vs Fleqsuvy in healthy adults with the same indication. The results showed similar bioavailability of baclofen at 20mg dose level for the oral suspension and oral tablet formulations in the fasting conditions
- Fleqsuvy is supplied as a 25mg/5mL (5mg/mL) oral grape-flavored suspension and is available in bottles of 120mL or 300mL
Pyrukynd
Active ingredient: Mitapivat Approved: February 21, 2022
Company: Agios Pharmaceuticals Disease: Hemolytic Anemia
- The approval was based on the P-III (ACTIVATE) & (ACTIVATE-T) studies to evaluate Pyrukynd vs PBO in patients with hemolytic anemia with PK deficiency. The therapy is expected to be available in the US in ~2wks.
- Both trials met their 1EPs i.e., In (ACTIVATE) trial, patients achieved a hemoglobin response (40% vs 0%), improvements for all pre-specified 2EPs including markers of hemolysis & ineffective erythropoiesis, changes in jaundice, tiredness & shortness of breath
- In (ACTIVATE-T) trial, 33% achieved a transfusion reduction, 22% were transfusion-free. The therapy is also under EMA’s review for PK deficiency while the regulatory decision in the EU is expected at end of 2022
Theragnostics’ NephroScan Receives the US FDA’s Approval for the Treatment of Kidney Disease
NephroScan
Active ingredient: technetium Tc 99m succimer injection Approved: February 23, 2022
Company: Theragnostics Disease: Kidney Disease
- The US FDA has approved NephroScan, a radiodiagnostic imaging drug that was used as an aid for the evaluation of renal parenchymal disorders in adult & pediatric patients including term neonates. The product was manufactured by ROTOP Pharmaka in Germany
- TC-99m DMSA imaging has been used to identify patients at risk for sequelae including hypertension, parenchymal scarring & chronic renal failure while radiologists also use Tc-99m DMSA imaging to split renal function, kidney shape, and position
- NephroScan is a sterile, single-dose kit used for the preparation of technetium Tc 99m succimer injection & marks the 1st US FDA therapy. GE Healthcare plans to distribute NephroScan in the US
Vonjo
Active ingredient: Approved: February 28, 2022
Company: CTI BioPharma Disease: Myelofibrosis and Thrombocytopenia
- The accelerated approval was based on the efficacy results from the P-III (PERSIST-2) study to evaluate Vonjo (200mg, bid, 400mg, qd) vs BAT in a ratio (1:1:1) in patients with myelofibrosis with a platelet count below 50 × 10^9/L
- The results showed that the patients treated with pacritinib 200mg, bid achieved a 29% reduction in spleen volume of at least 35% vs 3%. Additionally, the company is expected to complete (PACIFICA) trial in mid-2025
- Vonjo is an oral kinase inhibitor with specificity for JAK2 & IRAK1 without inhibiting JAK1. The company has launched patient support program, CTI Access to provides reimbursement & financial assistance programs for eligible patients
Related Post: Insights+: The US FDA New Drug Approvals in January 2022

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com














